Fc Fusion Protein Biosimilar Stable Cell Line Construction Service
Biosimilars are also named follow-up biologics, similar biological products, second entry biological, subsequent entry biological, biogenerics, and multisource products. They are highly similar to the licensed biological products and approved under specific regulatory measures. There is no clinically significant difference between these biological products and the reference ones in terms of purity, safety, and efficacy. Biologicals, produced by living cells or organisms, are composed of large and complex molecular entities that may be difficult to fully determine. Due to inherent variabilities of the biologic system and the manufacturing process, any resulting biological will reveal a certain degree of microheterogeneity. That is to say, the potential biosimilars, based on biopharmaceuticals that no longer under patent protection, are not generic equivalents of the originators, but a new therapeutic choice to treat several diseases.
Fc fusion proteins consist of Fc region of immunoglobin G (IgG) antibody (Hinge-CH2-CH3) and a desired linked protein. The Fc region binds to neonatal Fc receptor (FcRn) thereby rescuing it from degradation. The fused partner could be any other proteinaceous molecule of interest. Most frequently though, the linked protein has significant therapeutic potential and is attached to an Fc-domain to endow the final hybrid with additional beneficial pharmacodynamic and pharmacokinetic properties. The first therapeutic Fc fusion protein was indicated for the treatment of AIDS. Although therapeutic monoclonal antibodies (mAbs) are the top-selling biologics, the more application of Fc fusion proteins in the clinic is in progress. Currently, 11 Fc fusion proteins have been approved by the Food and Drug Administration (FDA) and there are several new Fc fusion proteins being in the pre-clinical and clinical development stage.
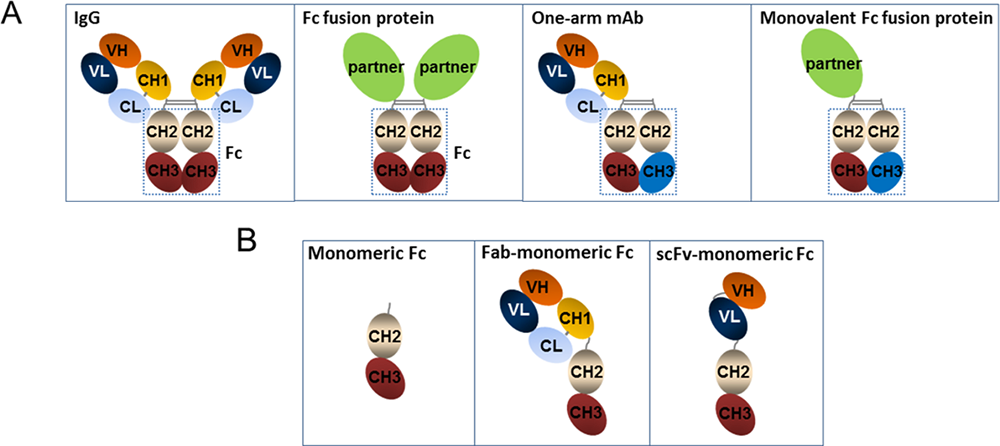
Fig.1 Structure of Fc fusion proteins.1
Data demands for the development and licensing of biosimilars are considerably rigorous than chemically synthesized small-molecule generics. For a generic product, the biological identification and display of a similar pharmacological profiling (bioequivalence) to the originator is usually sufficient to draw a conclusion on therapeutic equivalence. In contrast, biosimilars need to be developed relying on a more extensive comparison with the reference, to ensure close resemblance in physicochemical features, safety, and potency. Creative Biolabs has become an undisputed global service provider in the manufacture of various biologics. We are able to provide personalized services to assist customers in developing and producing biosimilars, including Fc fusion protein biosimilars, by a suit of stable cell lines. With the help of us, clients can obtain all-process technical interpretation, save project time and lower risks in studying biosimilars.
Reference:
-
Shan, Lu, et al. "Generation and characterization of an IgG4 monomeric Fc platform." PLoS One 11.8 (2016): e0160345. Distributed under Open Access license CC BY 4.0, without modification.
Related Sections
Services
Products
To discuss your Fc Fusion Protein Biosimilar Stable Cell Line Construction Service demands or to request a proposal, please contact us.
For Research Use Only. Not For Clinical Use.




