Cytokine Biosimilar Stable Cell Lines
A biosimilar is defined as a follow-up version of innovative medications that has been proved a high similarity to the reference already approved by the regulator. There are minor differences in clinically inactive components of a molecule which can be clinically meaningful, yet they do not substantially impact the high resemblance to the originator in their safety and efficacy. Biosimilars are intended to display comparable bioequivalence relative to reference biologicals and their definition makes it clear that they are not the exact copies or generic alternatives to the original drug, and thus are not currently considered interchangeable. As an expert in the field of drug development, Creative Biolabs provides custom services to construct stable cell lines for the production of biosimilars, including cytokines. With the help of us, clients can save project time and lower the risk of biosimilar developments.
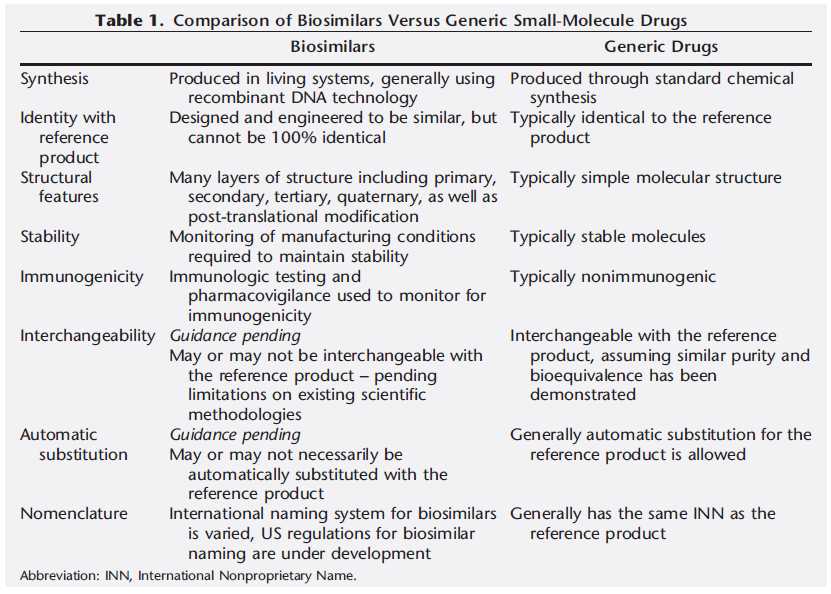
Table.1 Comparison of biosimilars versus generic small-molecule drugs. (Tkaczuk, et al., 2014)
Cytokines are small secreted proteins that enable receptor-mediated communication between cells and also message proteins that function as key modulators of immune responses by controlling proliferation, differentiation, and activation of different cell types. They coordinate innate immunity via inducing protective local inflammations and systemic acute phase responses and play a critical role in initiating, amplifying, directing, mediating, and regulating adaptive immunity. In turn, cytokines may cause tissue damage if they involve in autoimmunity or there are excessive responses occur. Under these situations, cytokines are deemed to potential therapeutic targets and cytokine-related drugs behave either by stimulating or blocking their activities. Today, hundreds of cytokines have been identified, and some of which have been used in therapeutic products, mostly for treating diseases, such as cancer, autoimmune, and viral infections.
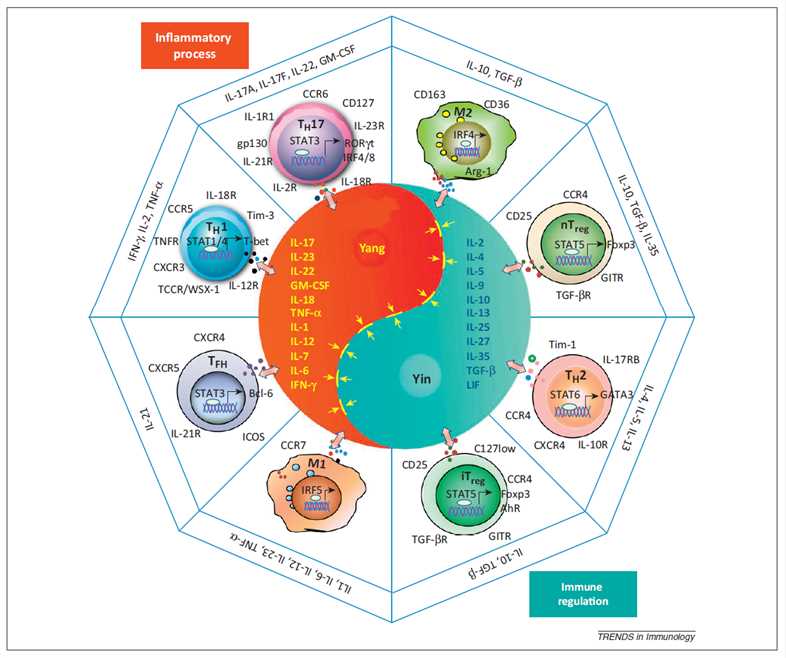
Fig.1 Interaction between inflammatory cells and cytokine milieu in Taoism. (Liu, et al., 2013)
The introduction of biosimilars brings promise to reduce the cost of therapy, and as the expiration of patents on chemically synthesized drugs is coming, their availability will further increase. Recently, there are new biological medicines, hormone biosimilars, being designed and produced for the treatment. In contrast to the first biotech drugs (e.g. insulin), biosimilars are much more structurally complex, and their manufacture processes are much more complicated. Biosimilars as the end products of such sophisticated technology are affected by many variables. There is no possibility to create biogenerics, but only biosimilar drugs. Furthermore, it is difficult for biosimilars to forego clinical trials in spite of their similarities to the reference molecules. Notably, Creative Biolabs has years of experience in drug development and undertakes varieties of assays. We would like to provide one-stop, personalized service packages for the cytokine biosimilars and other biological drugs through a set of stable cell lines.
References:
-
Rak Tkaczuk, K.H.; Jacobs, I.A. Biosimilars in oncology: from development to clinical practice. Semin Oncol. 2014, 41 Suppl 3: S3-S12.
-
Liu, X.; et al. Drug targets in the cytokine universe for autoimmune disease. Trends Immunol. 2013, 34(3): 120-128.
Related Sections
Services
Products
To discuss your Cytokine Biosimilar Stable Cell Lines demands or to request a proposal, please contact us.
For Research Use Only. Not For Clinical Use.




