Creative Biolabs now provides cGMP process for the manufacture of genetically CAR-T cells qualified for therapy. Based on our robust and scalable CAR-T cells manufacturing platform for product commercialization, Creative Biolabs can provide one-stop CAR-T cells manufacture services to meet our customers’ needs and keep adherence to cGMP and regulatory guidelines. These include enclosed system operations, robust process improvement, work flows simplification, process automation implementation, product testing and tracking, etc.
CAR-T cells are novel products that have unique characteristics that may impact product and clinical aspects of regulating these products. There are challenges with almost every aspect of the product development including CMC, patient monitoring, and trial design from eligibility to long term follow up. CAR-T cell science is a moving target and maintaining regulatory flexibility as knowledge improves is key to effective drug development.
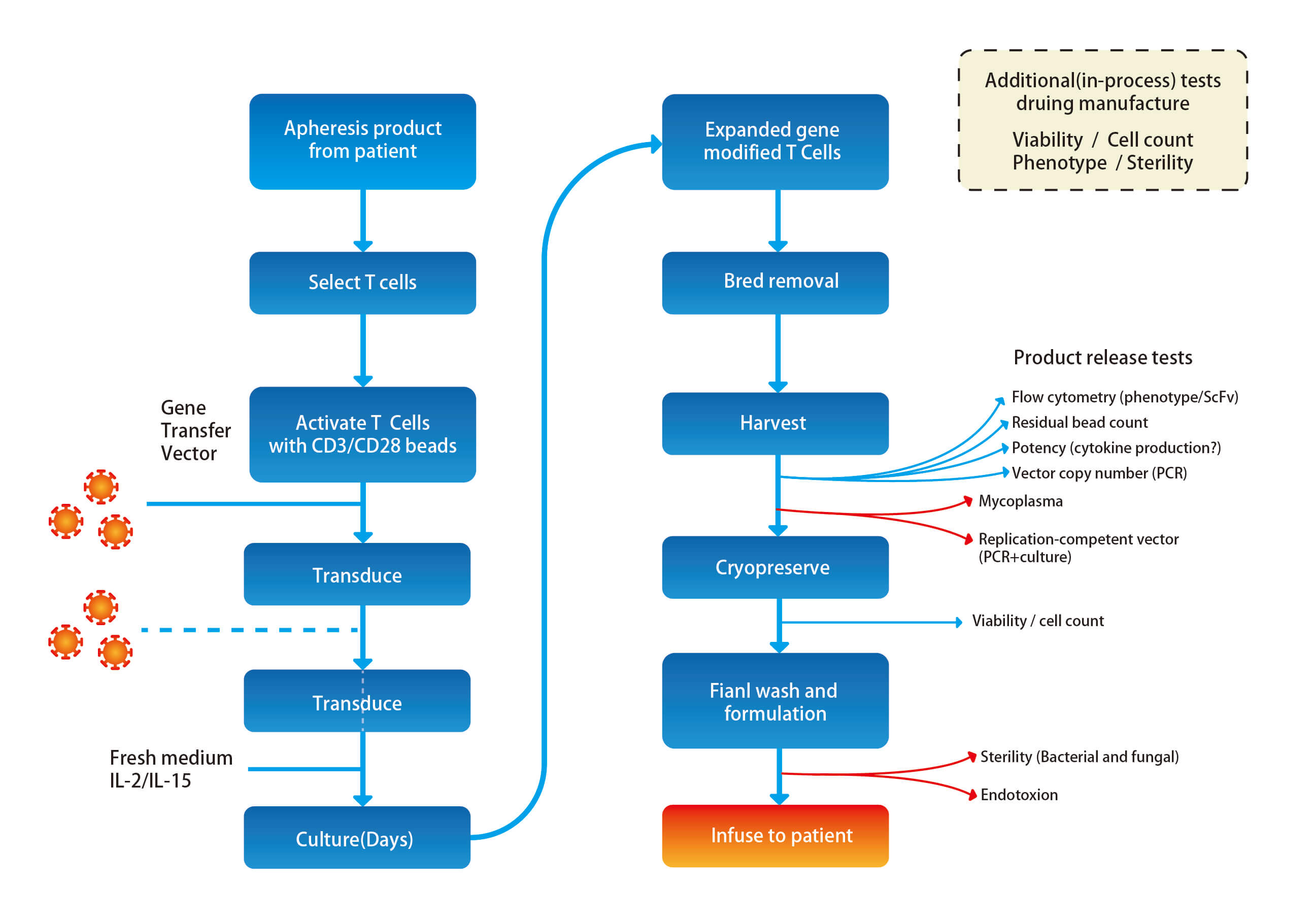
Figure. Product manufacture and testing
CAR-T treatment: current and future
• To date less than 1,000 patients treated globally
• High value products & clinical results have led to a movement from chemical drugs to biotherapeutics to ATMPs
• Huge increase in pharmaceutical interest
• Regulations have adapted to industry requirements, such as:
- ATMP regulations
- Multi product manufacturing
- Risk management principles (ICH Q9)
Reference:
-
Kaiser, A.D.; et al. Towards a commercial process for the manufacture of genetically modified T cells for therapy. Cancer gene therapy. 2015, 22(2): 72-78.
To discuss your CAR-T demands or to request a proposal, please contact us.
For Research Use Only. Not For Clinical Use.



