Antibody Biosimilar Stable Cell Lines
Biosimilars, usually referred to follow-on biologics, are intended to be a similar version of the originator drug that has already been marketed. Biosimilars are a type of complex proteins that distinct from those of small-molecule generics. Considering the patent expiry of biologics, biosimilars provide an attractively new commercial sight to a number of pharma companies. There are key-benefits where the main is that biosimilars could be alternative treatment modalities for patients who have not been offered treatments in the past. Moreover, regulatory agencies show a positive attitude to this concept and have launched the guidance and legislation for the development of biosimilars. As a prominent global service provider in antibody development, Creative Biolabs has strong capabilities to provide one-stop strategies to develop antibody biosimilars via a range of stable cell lines.
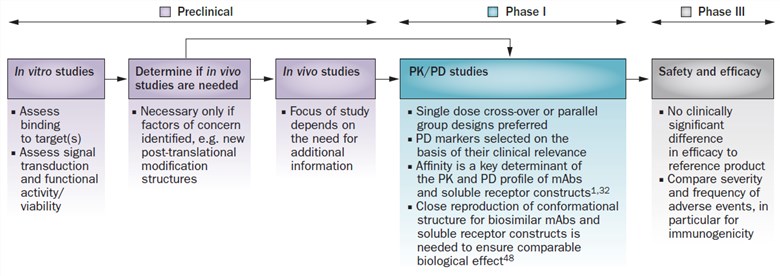
Fig.1 The European Medicines Agency (EMA) guidance on biosimilar mAbs: a stepwise approach. (Dörner & Kay, 2015)
In the past three decades, antibodies, particularly monoclonal antibodies (mAbs), have been exploited as one of the most significant perspectives of biotherapeutics for many diseases, such as cancer, inflammatory, autoimmune, and cardiovascular diseases. Likewise, numerous pharmaceutical companies initiated and accelerated the development of antibody biosimilars for their high value and the patent-expiration date of mAbs drugs. For the market approval of biosimilars, regulators demand high or fingerprinted similarity of biosimilar candidates to their innovative products, involving physicochemical properties, functional properties, safety profiling, and clinical efficiency. However, many posttranslational modifications (e.g. oxidation, glycosylation, deamidation) often occur during the expression processes of mAbs. Hence, the monitor, control, and characterization of antibody variants are essential for the similarity evaluation between biosimilar candidates and their reference medicines.
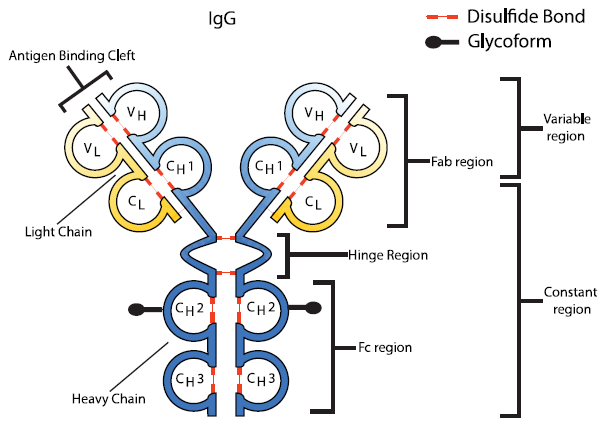
Fig.2 Basic structure of an IgG mAb. (Scott, et al., 2015)
Cell culture is of great importance during the process of development of therapeutic mAb drugs. The optimization of cell culture procedure contains the isolation and creation of high expressing cell lines and a robust growth condition with desired product quality. To achieve the goal, the regulation of glycosylation profile of mAbs has received a lot of attention since glycan structures on antibodies have substantial effects on bioactivities and clearance rates. Besides, some other critical quality attributes, such as aggregates, charge, and size variants are also dramatically influenced by the cell culture conditions. Remarkably, Creative Biolabs is skilled at utilizing orthometric in-process product quality control methods to evaluate the cell culture process and has established a variety of stable cell lines for antibody biosimilars. We would like to introduce our professional and systematic services to assist customers in biosimilar development requirements.
References:
-
Dörner, T.; Kay, J. Biosimilars in rheumatology: current perspectives and lessons learnt. Nat Rev Rheumatol. 2015, 11(12): 713-724.
-
Scott, B.J.; et al. Biosimilar monoclonal antibodies: A Canadian regulatory perspective on the assessment of clinically relevant differences and indication extrapolation. J Clin Pharmacol. 2015, 55 Suppl 3: S123-132.
Related Sections
Services
Products
To discuss your Antibody Biosimilar Stable Cell Lines demands or to request a proposal, please contact us.
For Research Use Only. Not For Clinical Use.




