Biosimilar Stable Cell Lines
The biosimilars are drugs that are highly similar but not identical to the biological reference. Their development and evaluation procedures are different from those of chemical generics. At Creative Biolabs, we focus on the challenges and opportunities of biosimilars and are committed to assisting clients in providing drug discovery, screening, optimization, and characterization profiling services. Increasingly, biosimilars are expected to reduce medical costs and offer more treatment options for patients worldwide.
Introduction
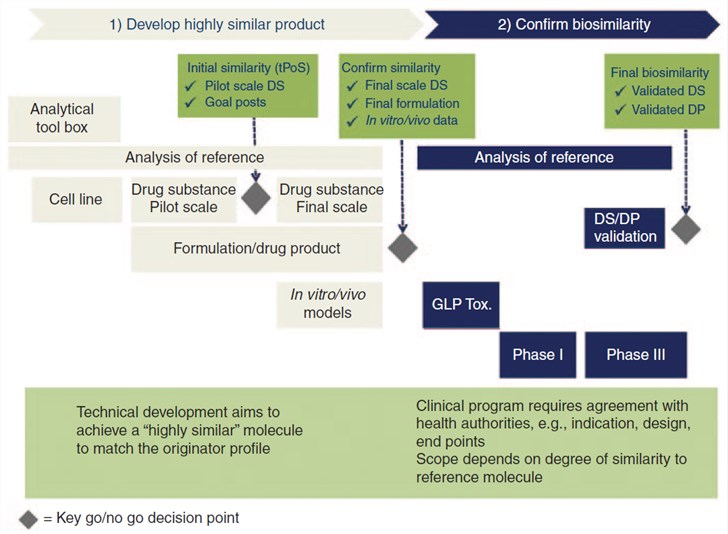
Fig.1 Biosimilar development requires a paradigm shift - the focus is on similarity and not on de novo efficacy. (McCamish & Woollett, 2013)
The biologic is a complex molecule in the living system. Its development involves multiple degrees of intricate, highly controlled manufacturing processes, combined with preclinical structural, biological, and functional assessments, as well as clinical safety and efficacy. Furthermore, to ensure a high level of similarity, a biosimilar undergoes a comparability exercise at every step of the development, testifying that potential differences from the reference are not clinically meaningful with regard to safety, purity, and potency [U.S. Food and Drug Administration (FDA)] or quality, safety, and efficacy [European Medicines Agency (EMA)]. Finally, clinical pharmacokinetic/pharmacodynamic (PK/PD), together with clinical efficacy/safety similarity, must be confirmed between biosimilar and originator. The regulators of FDA and the EMA will consider the totality of evidence from this comprehensive step-wise comparative similarity data in its determination of biosimilarity for licensing.
Biosimilars Development Guidelines
The commercial biosimilars are being used to optimize the clinically therapeutic strategies in medical oncology. A number of rigorous regulated processes required to develop a biosimilar have been designed to establish a high biosimilarity with a reference product in terms of structural, functional, biological, and clinical attributes. Based on the originator characterization, we can offer corresponding biosimilar developments from start to finish, from initial property to stability studies to determine a desirable biosimilar. In collaboration with our GMP partners, there would be the best versatile course of action for clients proposed biosimilar sooner.
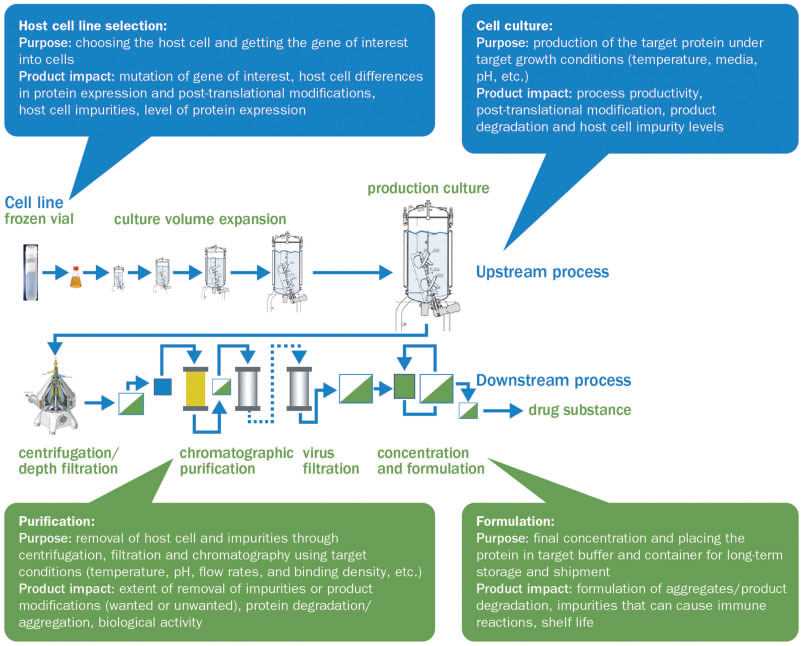
Fig.2 The biologics manufacturing process and the manufacturing steps that affect final characteristics of biologics. (Vulto & Jaquez, 2017)
Development & Analytical Services for Biosimilars
• Pre-consulting & GMP Manufacturing Processes.
• Drug Discovery: designing, screening, or modifying.
• Originator Product Characterization: HPLC, GC, MS, GC/MS, LC/MS/MS, ELISA, and biophysical techniques.
• Efficacy Studies: bioassay (in vivo & In vitro activity, enzyme assay), immunogenicity assay, binding assay, etc.
• Stability Studies: forced degradation, accelerated degradation, etc.
• Biosafety Evaluations: purity, dose, toxicity, etc.
• Lot Release and Comparability Testing.
• Early Phase Non-clinical & Clinical Pharmacology: PK/PD modeling, etc.
Besides, we also could provide regulatory affairs to deliver quality services that expedite drug development programs and help clients commercialize their outcomes with a short timeline.
Highlights:
• One-stop, custom-oriented service
• Guaranteed quality and reduced risk
• State-of-the-art technologies for similarity tests
• Time-saving and rational costs
• Timely report from feasibility analysis to final delivery
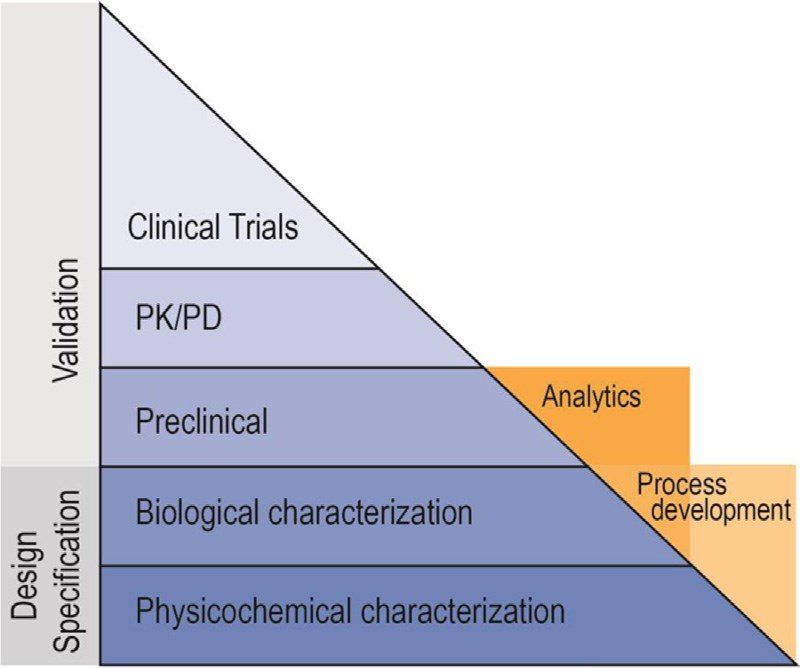
Fig.3 Relative data requirements for novel biosimilars. (Alten & Cronstein, 2015)
With the expiration of biological drug patents, biosimilars emerge and promise to be an attractive opportunity for physicians, patients, and healthcare organizations by offering greater choice and a more cost-effective alternative. Unlike biologics, the development of a biosimilar largely emphasizes nonclinical (physicochemical, biologic, and animal) aspects. At Creative Biolabs, our step-by-step approach for biosimilar development services could help clients move smoothly from product characterization and biosafety testing to comparability studies and clinical trials. If you have any interests, please contact us for more information.
Notably, we’d like to introduce our custom services of Biosimilar Stable Cell Lines in collaboration with downstream partners, including but not limited to:
References:
-
McCamish, M.; Woollett, G. The continuum of comparability extends to biosimilarity: how much is enough and what clinical data are necessary? Clinical Pharmacology & Therapeutics. 2013, 93(4): 315-317.
-
Vulto, A.G.; Jaquez, O.A. The process defines the product: what really matters in biosimilar design and production? Rheumatology. 2017, 56(suppl_4): iv14-iv29.
-
Alten, R.; Cronstein, B.N. Clinical trial development for biosimilars. Seminars in Arthritis and Rheumatism. WB Saunders. 2015, 44(6): S2-S8.
Related Sections
Services
Products
To discuss your Biosimilar Stable Cell Lines demands or to request a proposal, please contact us.
For Research Use Only. Not For Clinical Use.





